Difference between Elements and Atoms
Key difference: An element is a pure chemical substance that has one or one type of atom, distinguished by its atomic number. There are a total of 118 elements that have been identified, divided between metal, metalloids and non-metals. Each element has its own set of properties. Atoms are the basic units that all matter is made of. Each atom has a unique name, mass and size. The different types of atoms are called elements.
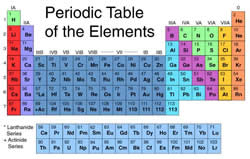 Elements and atoms are the some of the introductory terminology always used in chemistry. However, as science can get too complex, these terms can be easy to confuse.
Elements and atoms are the some of the introductory terminology always used in chemistry. However, as science can get too complex, these terms can be easy to confuse.
An element is a pure chemical substance that has one or one type of atom, distinguished by its atomic number. The atomic number is derived from the number of protons present in the element’s nucleus. There are a total of 118 elements that have been identified, divided between metal, metalloids and non-metals. Each element has its own set of properties. Most elements are available on the earth, while a few have been developed artificially through nuclear reactions. An element is already in its rawest form and cannot be further broken down. All of the elements can be found in the Periodic Table, listed by atomic number.
Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. They are so tiny that they cannot even be seen through a microscope. There are many types of atoms. Each atom has a unique name, mass and size. The different types of atoms are called elements.
Essentially, atom is a unit whereas elements are specific substances. An atom is the smallest possible units of that element that retains the properties of that element. For example: Iron (Fe) is an element. A block of iron can be broken down into iron flakes, which can then be further broken down into atoms. The atom would still exhibit all the properties of iron including magnetism.
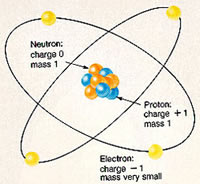 Each atom of any element is made up of protons, neutrons and electrons. The protons and neutrons make up the nucleus of the atom and are situated in the middle of the atom. The nucleus is surrounded by a cloud of electrons which are bound to the nucleus by an electromagnetic force. The electrons have a negative charge which is how they are attracted to the nucleus, as the protons in the nucleus have a positive charge. Neutrons, on the other hand, do not have a charge.
Each atom of any element is made up of protons, neutrons and electrons. The protons and neutrons make up the nucleus of the atom and are situated in the middle of the atom. The nucleus is surrounded by a cloud of electrons which are bound to the nucleus by an electromagnetic force. The electrons have a negative charge which is how they are attracted to the nucleus, as the protons in the nucleus have a positive charge. Neutrons, on the other hand, do not have a charge.
The number of protons, neutrons and electrons in an atom determine which element it is. For example: An atom of iron has 26 protons, 30 neutrons and 26 electrons. Each atom of iron will have this configuration.
Elements are the basic building blocks for all types of substances. They can combine with each other to form molecules. Atoms of different elements come together to make molecules. This happens via a chemical reaction. For example: two hydrogen atoms and one oxygen atom combine to make a water molecule.
Image Courtesy: universetoday.com, indiana.edu









Comments
Finally, a clear, complete description and comparison between atom and element. Great job!
Suzanne
Fri, 05/02/2014 - 02:18
Add new comment