Difference between Fission and Fusion
Key Difference: Fission and Fusion are two different kinds of nuclear reactions that produce energy, but they are opposite to each other. When an atom splits into two parts, either through natural decay or when initiated within a lab, it releases energy, this process is known as Fission. On the other hand, when two light atoms link together to make a heavier one, this process is known as Fusion.
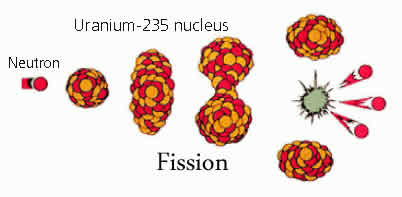 The two types of nuclear reactions used to produce energy are fission and fusion. According to Merriam Webster, Fission is “a process in which the nucleus of a heavy atom is split apart”. In simple terms, fission occurs when atom splits. It either happens through radioactive decay or because it has been bombarded by other subatomic particles known as ‘neutrinos’. Under unusual circumstances (the accomplishment of a "critical mass") the produced neutrons can split further atoms, which in turn bring about more splitting, creating a very fast chain reaction. Nuclear power plants exploit the process of fission to create energy.
The two types of nuclear reactions used to produce energy are fission and fusion. According to Merriam Webster, Fission is “a process in which the nucleus of a heavy atom is split apart”. In simple terms, fission occurs when atom splits. It either happens through radioactive decay or because it has been bombarded by other subatomic particles known as ‘neutrinos’. Under unusual circumstances (the accomplishment of a "critical mass") the produced neutrons can split further atoms, which in turn bring about more splitting, creating a very fast chain reaction. Nuclear power plants exploit the process of fission to create energy.
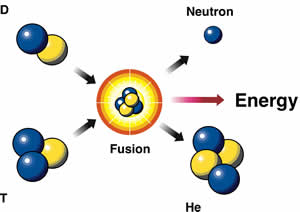 According to Merriam Webster, Fussion is “a process in which the nuclei of atoms are joined”. Extremely high threshold energy must be reached to combine atomic nuclei, and the temperature required is in the millions of degrees. The reaction releases huge amount of energy. This is the reaction that goes on continuously on the plane of sun which explains an unending source of power in the form of sun. Present researchers are using magnetic vacuum cavity and laser beams in an effort to produce the tremendous high-temperatures essential for the fusion procedure. If successful, the net energy gain would create a viable alternative energy option.
According to Merriam Webster, Fussion is “a process in which the nuclei of atoms are joined”. Extremely high threshold energy must be reached to combine atomic nuclei, and the temperature required is in the millions of degrees. The reaction releases huge amount of energy. This is the reaction that goes on continuously on the plane of sun which explains an unending source of power in the form of sun. Present researchers are using magnetic vacuum cavity and laser beams in an effort to produce the tremendous high-temperatures essential for the fusion procedure. If successful, the net energy gain would create a viable alternative energy option.
Comparison between Fission and Fusion:
|
|
Fission |
Fusion |
|
Definition |
In layman’s terms, fission is when an atom splits into two parts, either through natural decay or set of within a lab, when it releases energy. |
In layman’s terms, fusion occurs when two light atoms fuse together to make a heavier once. |
|
Major difference |
Fission breaks atomic nuclei into smaller pieces. |
Fusion joins atomic nuclei together. |
|
Elements |
The starting elements have a higher atomic number than that of the fission products. |
The element formed has more neutrons or more protons than that of the starting material. |
|
Process |
It is a process where unstable, heavy nucleus split into two lighter nuclei. |
It is a process where the two light nuclei combine together releasing vast amounts of energy. |
|
For example |
Uranium can fission to yield strontium and krypton. |
Hydrogen and hydrogen can fuse to form helium. |
Image Courtesy: hko.gov.hk, iter.rma.ac.be









Add new comment