Difference between Coagulation and Flocculation
Key Difference: Coagulation means to curdle; it basically refers to a chemical process in which the destabilization of non-settleable particles takes place. These particles form clumps with the help of a coagulant. On the other hand, flocculation means to form flocs. It can be described as a physical or a mechanical process in which the coagulated clumps or flocs are joined together to form masses as a cloud and then a precipitate.
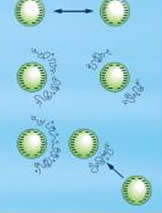 In coagulation, the forces responsible for keeping the particles apart after they contact, are reduced. Flocculation brings the de-established colloidal particles together and they form large aggregates. Both these techniques are employed in the treatment of water. Thus, we can understand the process of coagulation and flocculation with the help of examples derived from water treatment techniques.
In coagulation, the forces responsible for keeping the particles apart after they contact, are reduced. Flocculation brings the de-established colloidal particles together and they form large aggregates. Both these techniques are employed in the treatment of water. Thus, we can understand the process of coagulation and flocculation with the help of examples derived from water treatment techniques.
Water may contain colloidal solids like non-settleable organic matter, clay particles, bacteria, plankton, small particles of decayed plant material, etc. Thus, coagulation and flocculation techniques are employed for separating these impurities from water.
Coagulation is achieved by neutralizing the particles and thus, the repelling force between the particles is greatly reduced. After employing the flocculation process, the coagulated particles form a large agglomeration, which is also known as floc.
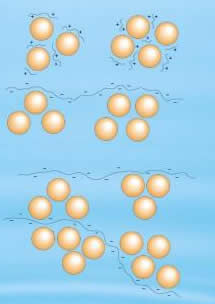
In flocculation, these agglomerations of destabilized particles take the form of large particles. This can also be achieved by adding high molecular weight, water soluble organic polymers. Due to these polymers, the size of the floc increases and then the particles settle down. It is very important to gently mix the flocculating agent at a slow speed so that small flocs can easily agglomerate into large particles, and finally settle down.
For water treatment, coagulation is generally followed by flocculation.
Thus, in terms of water treatment, both can be differentiated easily. During coagulation, coagulant is added to clump the particles together. On the other hand, during flocculation, the solution is mixed gently, so that the small clumps formed during coagulation, gather together and form larger clumps. These large clumps easily settle down and thus can be separated.
Images Courtesy: sswm.info








Comments
veryyyyy nice article
simran
Sat, 06/14/2014 - 19:58
I like chicken
Derrick Donnerham
Tue, 04/29/2014 - 08:37
very nice and easy to understad
jeetendra mishr...
Wed, 03/26/2014 - 12:21
Thank you very much. This article explains it very simple manner.
Learner
Mon, 03/10/2014 - 17:06
Nice and simple article.But one necessary thing is missing that is the mixing of lime to increase the toughness and density of newly generated flocs.
Atif Awan
Sat, 03/01/2014 - 10:15
Pages
Add new comment