Difference between Oxidation and Reduction
Key difference: Oxidation and reduction are two processes that occur in a redox reaction. In oxidation, a molecule, atom, or ion experiences an increase in oxidation state or basically, it looses electrons. In reduction, a molecule, atom, or ion experiences a decrease in oxidation state, or rather it gains electrons.
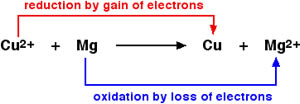 Oxidation and reduction are two processes that occur in a redox reaction. Redox stands for reduction-oxidation. It is essentially a reaction, in which ‘atoms have their oxidation state changed’. An easier way to say this is that the reaction involves the transfer of electrons between two or more molecules.
Oxidation and reduction are two processes that occur in a redox reaction. Redox stands for reduction-oxidation. It is essentially a reaction, in which ‘atoms have their oxidation state changed’. An easier way to say this is that the reaction involves the transfer of electrons between two or more molecules.
In oxidation, a molecule, atom, or ion experiences an increase in oxidation state or basically, it looses electrons. Reduction is essentially the opposite of that. In reduction, a molecule, atom, or ion experiences a decrease in oxidation state, or rather it gains electrons.
Oxidation and reduction always occur simultaneously. This is mainly because when the molecule, atom, or ion looses an electron; the electron must go somewhere; i.e. to another molecule, atom, or ion. This molecule, atom, or ion, in turn gains the electron that was lost.
Let us consider an example:
H2 + F2 → 2 HF
Let us break these down to atoms and ions:
H2 → 2 H+ + 2 e−
The hydrogen molecule looses two electrons to become two hydrogen ions. This is essentially the oxidation reaction, as it involved the loss of electrons.
F2 + 2 e− → 2 F−
The fluorine gains the two electrons lost by the hydrogen molecule to become two fluorine ions. This is essentially the reductions reaction, as it involved the gain of electrons.
2 H+ + 2 F− → 2 HF
 The two hydrogen ions and the two fluorine ions come together to form two molecules of hydrogen fluoride.
The two hydrogen ions and the two fluorine ions come together to form two molecules of hydrogen fluoride.
Hence, the entire redox reactions can be written as:
H2 + F2 → 2 HF
While, oxidation and reduction occur on a molecule or atom level, it is still possible to see examples redox reactions in our daily lives. Some examples of a redox reaction are the rusting of iron; the browning of an apple; bleach breaking down stains, etc.
Image Courtesy: chemguide.co.uk, amazingrust.com









Comments
good articles
authentic gucci...
Fri, 01/30/2015 - 16:08
Add new comment