Difference between Purine and Pyrimidine
Key Difference: Pyrimidine is a heterocyclic aromatic organic compound composed of nitrogen and carbon. Purine is also a heterocyclic aromatic organic compound composed of a pyrimidine ring fused to an imidazole ring. Pryimidine bases are composed of a single ring structure, whereas Purines consist of fused double ring. They differ in many aspects like melting point, boiling point, etc.
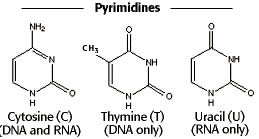 Pyrimidine is a heterocyclic or six membered nitrogen heterocyclic compounds which possesses the molecular formula C4H4N2. Heterocyclic compounds refer to the organic compounds which possess a ring structure containing atoms like sulfur, oxygen or nitrogen. Pyrimidines are available in nature in various forms.
Pyrimidine is a heterocyclic or six membered nitrogen heterocyclic compounds which possesses the molecular formula C4H4N2. Heterocyclic compounds refer to the organic compounds which possess a ring structure containing atoms like sulfur, oxygen or nitrogen. Pyrimidines are available in nature in various forms.
The word pyrimidine has been coined by Pinner in 1884, who combined the words pyridine and amidine to form pyrimidine; on the basis of the structural similarity to those compounds. It contains two nitrogen atoms at first and third positions of the sex membered ring.
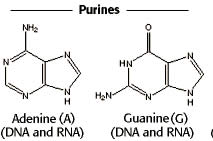 Purine refers to a group of heterocyclic compounds which is composed of a two ring structure made up of carbon and nitrogen atoms. A purine is a heterocyclic organic compound consisting of a pyrimidine ring fused to an imidazole ring. The term purine has been coined by Emil Fischer in 1884. Like Pyrimidine it also forms a group of nitrogenous bases. They are components of nucleic acids.
Purine refers to a group of heterocyclic compounds which is composed of a two ring structure made up of carbon and nitrogen atoms. A purine is a heterocyclic organic compound consisting of a pyrimidine ring fused to an imidazole ring. The term purine has been coined by Emil Fischer in 1884. Like Pyrimidine it also forms a group of nitrogenous bases. They are components of nucleic acids.
Pyrimidines and Purines are two different groups of organic bases. Pyrimidines tends to be smaller in comparison to Purines, as Pyrimidines contain a single ringed structure and Purines possess a double ring structure.
Pyrimidine and purine bases are very important for life as they are included in the structures of DNA and RNA. Both belong to the kingdom of Organic Compounds and superclass Aromatic Heteropolycyclic Compounds. Pyrimidines belong to the class of Diazines, whereas Purines belong to the class Imadazopyrimidines. Purines molecules are complex and heavier than molecules of pyrimidines, and therefore possess high melting and boiling points than pyrimidines.
Comparison between Purine and Pyrimidine:
|
|
Pyrimidines |
Purines |
|
Definitions |
Pyrimidine is a heterocyclic aromatic organic compound composed of nitrogen and carbon. |
Purine refers to a group of heterocyclic compounds which is composed of a two ring structure made up of carbon and nitrogen atoms |
|
Example Nucleobases |
Thymine, Uracil and Cytosine |
Adenine and Guanine |
|
Structure |
Contains1 carbon-nitrogen ring and 2 nitrogen atoms |
Consists of a pyrimidine ring fused to a imidazole ring. Contains two carbon nitrogen rings and four nitrogen atoms. |
|
Molecular Formula |
C4H4N2 |
C5H4N4 |
|
Melting Point and Boiling Point |
Comparatively lower melting and boiling points |
Comparatively high melting and boiling points |
|
Function as precursor molecules |
Not known to function as precursor molecules |
Function as precursor molecules in the synthesis of chemical compounds like thephylline |
Images Courtesy: alevelnotes.com









Comments
what are the nitrogenous base of purine and pyramidine?
Anonymous
Fri, 08/07/2015 - 07:22
Add new comment