Difference between Intensive and Extensive Properties
Key Difference: Intensive properties refer to properties that are independent compared to the size or quantity of the substance. Extensive properties refer to properties that are dependent on the size or quantity of the substance.
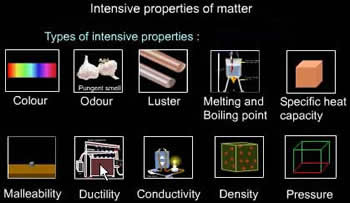 Intensive and extensive are properties of matter that are used in basic chemistry. It is one of the most important used tools when trying to determine a new element. These two terms are also commonly used in thermodynamics and materials science. The terms refer to the dependency of the properties on the size or the quantity of the substance. This distinction is made to understand if the substance changes when more of it is added or some of it is removed from the whole.
Intensive and extensive are properties of matter that are used in basic chemistry. It is one of the most important used tools when trying to determine a new element. These two terms are also commonly used in thermodynamics and materials science. The terms refer to the dependency of the properties on the size or the quantity of the substance. This distinction is made to understand if the substance changes when more of it is added or some of it is removed from the whole.
Intensive properties refer to properties that are independent compared to the size or quantity of the substance. These properties do not change when more of a substance is added or some of the substance is removed. Intensive properties include: density, color, viscosity, electrical resistivity, spectral absorption, hardness, melting point/boiling point, pressure, ductility, elasticity, malleability, magnetization, concentration, temperature and magnetic field.
These properties do not change if the size of the quantity of the substance changes. For example: the hardness of a diamond does not change, no matter how many times the diamond is cut. The color of the salt does not change no matter how much of it is added to the original amount. These all describe the intensive properties of the diamond and salt.
 Extensive properties refer to properties that are dependent on the size or quantity of the substance. These properties change depending on how much of the substance is added or removed. The value of the additive property is proportional to the size of the system. For example if the size is increased, then the property will also increase. Extensive properties include: energy, entropy, mass, length, particle number, number of moles, volume, magnetic moment, weight and electrical charge.
Extensive properties refer to properties that are dependent on the size or quantity of the substance. These properties change depending on how much of the substance is added or removed. The value of the additive property is proportional to the size of the system. For example if the size is increased, then the property will also increase. Extensive properties include: energy, entropy, mass, length, particle number, number of moles, volume, magnetic moment, weight and electrical charge.
These properties are directly proportional to the size and the quantity of the substance. For example: if the amount of water increases, the weight of the water will also increase; the more the water, the heavier it will be. Another example: the energy it would take to melt an ice cube is proportional to its size. The energy it would take to melt and ice cube differs from the energy that would be required to melt an iceberg.
Image Courtesy: goalfinder.com, 2012books.lardbucket.org








Comments
It is a very nice word.
Kadagathur Rahu...
Tue, 08/12/2014 - 21:49
Add new comment