Difference between Nuclear Fission and Nuclear Fusion
Key Difference: Nuclear fission and fusion are two nuclear processes or reactions in which energy is released. Nuclear fusion takes place by the combination of light nuclei like deuterium and tritium. On the other hand in nuclear fission, a nucleus like Uranium-235 and Plutonium-239 splits into lighter nuclei. Fission is comparatively easy to achieve than fusion. However, fusion emits more energy than fission.
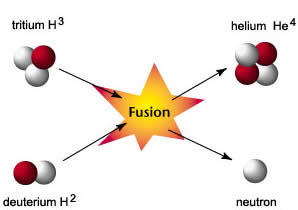 Nuclear fusion reaction involves the process of combining two atoms or more together and forming newer ones. It happens with respect to the nuclei, as they collide and fuse together in order to form the nuclei of heavier elements. This process generates a lot of energy. The most common example of fusion reaction is the fusion of hydrogen taking place in the sun.
Nuclear fusion reaction involves the process of combining two atoms or more together and forming newer ones. It happens with respect to the nuclei, as they collide and fuse together in order to form the nuclei of heavier elements. This process generates a lot of energy. The most common example of fusion reaction is the fusion of hydrogen taking place in the sun.
Nuclear fusion is carried out with the aid of extremely high temperatures. These temperatures are not easy to achieve. Apart from this, a lot of safety concern is required to handle the released hot gases. Nuclear fusion occurs naturally in stars. However, in fusion bombs it is started off by a fission bomb.
 Nuclear fission is a process in which nuclei of heavy elements divides into nuclei of light elements. The nucleus of the element breaks down into two parts. Fission takes place when the electrical forces are able to overcome the nuclear forces and the nucleus splits.
Nuclear fission is a process in which nuclei of heavy elements divides into nuclei of light elements. The nucleus of the element breaks down into two parts. Fission takes place when the electrical forces are able to overcome the nuclear forces and the nucleus splits.
Fission takes place when a large isotope is bombarded with a neutron. Due to this collision, this large isotope splits into two or more elements. In Fission, neutrons are also released along with energy. These neutrons further split more nuclei and a series or a chain reaction takes place.
Nuclear fission and fusion can be considered as just the two opposite reactions. However, both release energy. Nuclear fission can take place in room temperature. However, fusion can only be attained at a very high temperature. The amount of energy released is enormous in the case of fusion. Unlike fission, fusion does not exhibit any type of chain reaction.
Comparison between Nuclear Fission and Nuclear Fusion:
|
|
Nuclear Fission |
Nuclear Fusion |
|
Definition |
In nuclear fission, a heavy nucleus like Uranium-235 and Plutonium-239 splits into light nuclei. |
Nuclear fusion takes place by the combination of light nuclei like deuterium and tritium and producing heavy nuclei. |
|
Easy to achieve |
Comparatively easy to achieve |
Comparatively difficult to achieve |
|
Amount of energy released |
Comparatively low |
Comparatively high |
|
Example |
Uranium-235 is bombarded with a slow neutron, and it temporarily transforms into a very unstable isotope, uranium-236 |
In a hydrogen bomb, two isotopes of hydrogen, deuterium and tritium combine and form nucleus of helium and a neutron. This fusion releases 17.6 MeV of energy. |
|
Temperature requirement |
Takes place at room temperature |
Requires a very high temperature nearly equal to 4 * 10^6 degree centigrade |
|
Energy utilization |
These reactions are controllable, and therefore can be used for generating electricity |
These reactions cannot be controlled, and therefore, the energy released cannot be used for generating electricity |
|
Type of reaction |
It gives rise to a chain reaction |
It is not a chain reaction |
| Example is equation |  |
.jpg) |
Image Courtesy: atomicarchive.com, chemistry.tutorvista.com








Comments
very nice ,useful to school students. figures in table is need
DHAMODHARAN
Thu, 04/24/2014 - 11:27
Add new comment