Difference between Adsorption and Absorption
Key Difference: Absorption is a bulk phenomenon in which an absorbate completely penetrates into the body of a solid or liquid to form a compound or a solution. On the other hand, adsorption is a surface phenomenon in which molecules of an adsorbate concentrates only on the surface of an adsorbent.
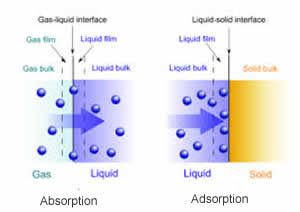 Many people consider absorption and adsorption as the same. However, both are different from each other. Absorption refers to a process in which molecules of a substance penetrate into the bulk of a solid, or liquid materials to form solutions or compounds. In simple language, it depicts the penetration of one substance into another in a complete manner. The molecules or atoms of a substance gets totally penetrated into the volume of the other molecules. It is sucked that much that it literally becomes a part of the other substance. It can either be a chemical reaction or a physical process. Generally, absorptions occur by the process of diffusion.
Many people consider absorption and adsorption as the same. However, both are different from each other. Absorption refers to a process in which molecules of a substance penetrate into the bulk of a solid, or liquid materials to form solutions or compounds. In simple language, it depicts the penetration of one substance into another in a complete manner. The molecules or atoms of a substance gets totally penetrated into the volume of the other molecules. It is sucked that much that it literally becomes a part of the other substance. It can either be a chemical reaction or a physical process. Generally, absorptions occur by the process of diffusion.
Chemical Reaction – carbon dioxide absorbed by a solution of potassium carbon
Physical Process – Air absorbed in water by dissolving
 On the other hand, adsorption deals with only the adhesion on the surface. It is referred to a process by which the molecules of a gas or liquid solute stick only at the surface a solid or a liquid (adsorbent). It results into higher concentration of adsorbate molecules on the surface. Unlike, absorption, they are not totally consumed by the other substance. It can be simply referred as the process by which molecules collect at the surface of the substance.
On the other hand, adsorption deals with only the adhesion on the surface. It is referred to a process by which the molecules of a gas or liquid solute stick only at the surface a solid or a liquid (adsorbent). It results into higher concentration of adsorbate molecules on the surface. Unlike, absorption, they are not totally consumed by the other substance. It can be simply referred as the process by which molecules collect at the surface of the substance.
There are two types of adsorption modes – chemical and physical. In chemical adsorption the molecules and surface are bonded by weak Vander Walls forces. On the other hand, in chemical adsorption, a chemical bond is formed between molecules and surface.
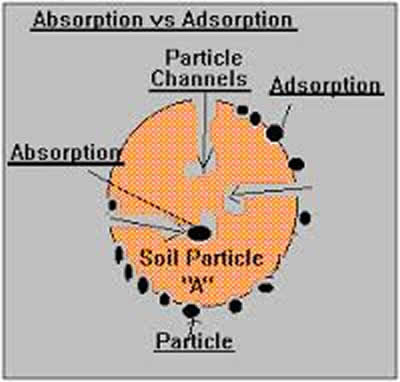
Therefore, the primary difference between adsorption and absorption is that absorption is a bulk phenomenon which means that it happens throughout the body of the material, whereas adsorption remains to be a surface phenomenon. Adsorption is always exothermic, whereas absorption is endothermic. Sorption includes both processes of absorption and adsorption.
Comparison between Adsorption and Absorption:
|
|
Adsorption |
Absorption |
|
Defintion |
Accumulation of a gas or liquid solute on the surface of a solid or a liquid |
Diffusion of a substance into a liquid or solid to form a solution or compound |
|
Example |
Inert gases are adsorbed on charcoal. |
A dry sponge absorbs water |
|
Heat exchange |
Exothermic with exception of adsorption of H2 on glass |
Endothermic |
|
Attaining Equilibrium |
Comparatively faster |
Comparatively slowly |
|
Concentration |
Concentration on the surface of the adsorbent is different from than in the bulk |
Concentration remains the same throughout the material |
|
Rate of occurrence |
It is rapid initially but later its rate starts to decline |
It takes place at uniform rate |
Image Courtesy: wikipedia.org.com, webapps.cee.vt.edu









Add new comment