Difference between Alpha, Beta and Gamma Radiation
Key Difference: Alpha radiation can be described as the producer of high energy and fast moving helium particles. Beta radiation is the producer of fast moving electrons and can penetrate further in comparison to the alpha particles. Gamma radiations are high energy radiations that are in the form of electromagnetic waves, and these radiations do not give off any particle like alpha and gamma radiations.
Radiation is an energy that emits from the source and then it travels through some material or space. Some examples of radiations are light, heat and sound. These radiations are absorbed in their path by substances. The intensity of radiation reduces with respect to the distance from the source radioactive material.
The radiations can be primarily divided into three types of radiation.
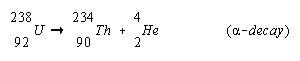 Alpha- These can also be referred to alpha particles that are emitted in the alpha decay. An alpha particle can be referred to as a Helium atom containing two neutrons and two protons. When an alpha particle is emitted from the nucleus then two units of atomic number and four units of mass number are decreased. These radiations are not able to penetrate skin, but the materials emitting these kinds of radiations can be harmful to human, in case they are inhaled, swallowed or absorbed through open wounds. Examples of some alpha emitters: radium, radon, uranium, thorium, etc.
Alpha- These can also be referred to alpha particles that are emitted in the alpha decay. An alpha particle can be referred to as a Helium atom containing two neutrons and two protons. When an alpha particle is emitted from the nucleus then two units of atomic number and four units of mass number are decreased. These radiations are not able to penetrate skin, but the materials emitting these kinds of radiations can be harmful to human, in case they are inhaled, swallowed or absorbed through open wounds. Examples of some alpha emitters: radium, radon, uranium, thorium, etc.
Beta- These can be referred to as beta particles. Any nucleus that contains an unstable ratio of neutron to protons may decay and emit the electrons known as beta particles. Due to the 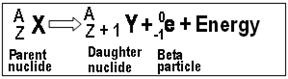 emission, net change of one unit takes place in the atomic number. These particles are negatively charged. They are fast moving particles and that too with lots of energy. A beta particle is about 8000 times smaller than compared to an alpha particle. Due to this smaller size, they are able to penetrate clothes as well as skin. Examples of some pure beta emitters: strontium-90, carbon-14, tritium, and sulfur-35.
emission, net change of one unit takes place in the atomic number. These particles are negatively charged. They are fast moving particles and that too with lots of energy. A beta particle is about 8000 times smaller than compared to an alpha particle. Due to this smaller size, they are able to penetrate clothes as well as skin. Examples of some pure beta emitters: strontium-90, carbon-14, tritium, and sulfur-35.
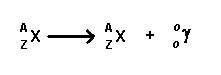 Gamma- These are extremely high energy photons that can travel through various matters. The emission of these rays does not change the number of protons or neutrons in the nucleus. They affect the energy states of the nucleus by moving them from a higher or unstable energy state to lower or stable energy state. This is due to the fact that they have no mass of their own. These rays can pass through the entire body and thus, they can affect all the tissues of the skin. These rays are quite similar to x-rays. Examples of some gamma emitters: iodine-131, cesium-137, cobalt-60, radium-226, and technetium-99m.
Gamma- These are extremely high energy photons that can travel through various matters. The emission of these rays does not change the number of protons or neutrons in the nucleus. They affect the energy states of the nucleus by moving them from a higher or unstable energy state to lower or stable energy state. This is due to the fact that they have no mass of their own. These rays can pass through the entire body and thus, they can affect all the tissues of the skin. These rays are quite similar to x-rays. Examples of some gamma emitters: iodine-131, cesium-137, cobalt-60, radium-226, and technetium-99m.
Alpha and beta radiations are in the forms of particles, whereas gamma radiations are in the form of electromagnetic rays.
Some of the differences are listed below in the table:-
|
|
Alpha |
Beta |
Gamma |
|
Definition |
Alpha particles can be referred to as a Helium atom containing two neutrons and two protons. |
Any nucleus that contains an unstable ratio of neutron to protons may decay and emit the electrons known as beta particle. |
These are extremely high energy photons that can travel through various matters. |
|
Penetration power |
Least (can be stopped or absorbed by a sheet of paper) |
Can penetrate air and paper (stopped by a thin sheet of aluminum) |
Most penetrating (higher levels can only be stopped by many centimeters of lead or many meters of concrete) |
|
Mass and charge |
Biggest |
Middle values |
Smallest |
|
Ionizing power (ability to remove electrons from atoms to form positive ions) |
Very high |
Moderate |
Lowest |
|
Presence detector |
A thin-window Geiger-Mueller (GM) probe can detect the presence of alpha radiation. |
Beta emitters can be detected with a survey instrument and a thin-window GM probe. |
Gamma rays can be detected by survey meters with a sodium iodide detector probe. |
|
Symbol |
α |
β, e |
γ |
|
Charge value |
+2e |
-e |
0 |
|
Speed |
~5% speed of light |
up to 98% the speed of light |
speed of light |
|
Affected by electric and magnetic fields |
Yes |
Yes |
No |
|
Distance traveled |
2-4 cm |
2-3 m |
500 m |
Images Courtesy: physick.wikispaces.com







Comments
Confused radiog...
Wed, 11/15/2017 - 07:25
Add new comment