Difference between Diffusion and Osmosis
Key Difference: Diffusion is the process by which molecules move and travel from one place to another without requiring bulk motion. Diffusion results in molecules moving or mixing by only using kinetic energy. Osmosis is a type of diffusion, where molecules mix through a semipermeable membrane to a more concentrated solution from a more dilute solution.
Diffusion and Osmosis are important concepts that play a huge part in our sustenance. Both of these terms refer to the movement of molecules and particles all around us. They play an active role in many fields such as physics, chemistry and biology. Diffusion is also used in sociology, economics and finance to refer to the diffusion of people, ideas and values. Though both refer to the movement of molecules and particles, they differ from each other and should not be used interchangeably.
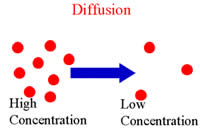 Diffusion is the process by which molecules move and travel from one place to another without requiring bulk motion. Diffusion results in molecules moving or mixing by only using kinetic energy. The word ‘diffusion’ is derived from the Latin word "diffundere" meaning "to spread out". In diffusion, molecules are in a constant state of movement and when propelled by kinetic or thermal energy they tend to mix with other molecules resulting in an inseparable mixture. Let’s take a practical approach, one container is divided in A & B sections using a solid partition; the first section is filled with water, while the second section is filled with red dye. Now, when the partition is lifted the dye and water try to fill the whole container. Then the dye slowly taints the water red, this being diffusion.
Diffusion is the process by which molecules move and travel from one place to another without requiring bulk motion. Diffusion results in molecules moving or mixing by only using kinetic energy. The word ‘diffusion’ is derived from the Latin word "diffundere" meaning "to spread out". In diffusion, molecules are in a constant state of movement and when propelled by kinetic or thermal energy they tend to mix with other molecules resulting in an inseparable mixture. Let’s take a practical approach, one container is divided in A & B sections using a solid partition; the first section is filled with water, while the second section is filled with red dye. Now, when the partition is lifted the dye and water try to fill the whole container. Then the dye slowly taints the water red, this being diffusion.
Diffusion causes molecules to shift from a higher concentration area to a lower concentration area, resulting in the mix of all the molecules. Diffusion stops when all the molecules are evenly spread out. Diffusion is not limited to water and works best in gaseous states, where molecules have more energy and ability to mix with other molecules. There are two approaches to diffusion: phenomenological and atomistic. According to the phenomenological approach the molecules travel from regions of higher concentration to regions of lower concentration. In the atomistic approach, diffusion is considered to happen due to random walk of the diffusing particles, in which the diffusion is propelled by thermal energy causing them to mix together. Diffusion plays an important part in creating minerals, nutrients and energy that are required by the body.
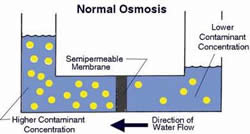 Osmosis is a type of diffusion, where molecules mix through a semipermeable membrane to a more concentrated solution from a more dilute solution. A semipermeable membrane is a barrier that only allows certain substances to pass through blocking all others. A cell wall is a semipermeable membrane as it allows water and certain substances diluted in water to pass through. Osmosis requires water in order to be able to pass through the membrane. The transport of substances is also to equalize the solution concentration on both sides of the membrane. Osmosis can also refer to a physical process in which solvents moves across a semipermeable membrane, which effectively separates the solvent from the solute resulting in two different solutions of different concentrations. This is known as reverse osmosis.
Osmosis is a type of diffusion, where molecules mix through a semipermeable membrane to a more concentrated solution from a more dilute solution. A semipermeable membrane is a barrier that only allows certain substances to pass through blocking all others. A cell wall is a semipermeable membrane as it allows water and certain substances diluted in water to pass through. Osmosis requires water in order to be able to pass through the membrane. The transport of substances is also to equalize the solution concentration on both sides of the membrane. Osmosis can also refer to a physical process in which solvents moves across a semipermeable membrane, which effectively separates the solvent from the solute resulting in two different solutions of different concentrations. This is known as reverse osmosis.
Osmosis is divided into three stages: hypotonic, isotonic and hypertonic. In hypotonic, the external solution is less concentrated than the internal solution, while in hypertonic, the external solution is more concentrated than the internal solution. Isotonic is the equilibrium that is reached when both the solutions have the same concentration. Osmosis is used by various cells and organisms in order to achieve equilibrium. Cells also use osmosis in order to acquire nutrients and energy form water-based solutions into the cell. The cell membranes act as a semipermeable passage, allowing large and polar molecules, such as ions, proteins, and polysaccharides to pass through while blocking non-polar and/or hydrophobic molecules such as lipids, small molecules like oxygen, carbon dioxide, nitrogen, nitric oxide, etc. The permeability of the cell membrane depends on solubility, charge, or chemistry, as well as solute size.
The word "osmosis" is derived from words "endosmose" and "exosmose", coined by French physician René Joachim Henri Dutrochet. A practical example of osmosis is a cell, when placed in a high concentrated solution (salt water), will release water from the cell to the medium around it, resulting it in shrinking. However, if it is placed in a lower concentration solution such as fresh water, the cell will absorb the water and grow bigger. Osmosis is also the primary way that plants acquire water and necessary nutrients for survival from the ground. The process of absorbing water from the soil is osmosis.
|
|
Diffusion |
Osmosis |
|
Definition |
Diffusion the movement of particles or molecules from a high concentration area to a low concentration area. |
Osmosis is the movement of particles or solvent molecules through a partially permeable membrane into a high concentration area from a low concentration area. |
|
Process |
Diffusion commonly occurs in molecules that are in gaseous state or within gas molecules and liquid molecules. The gases constantly collide with the membrane, which when removed, will allow the molecules to move freely. |
Osmosis occurs primarily with water and cells. If the medium that surrounds the cell has higher water concentration, the cell will absorb the water. |
|
Importance |
Diffusion allows for creation of important nutrients and energy required by the body. |
Osmosis allows cells to absorb many nutrients that are available in the water. |
|
Water |
Does not require water. |
Requires water. |
|
Concentration Gradient |
Goes from a high concentration gradient to a low concentration gradient |
Moves down concentration gradient |
|
Energy |
Passive, as no external energy is needed. |
Passive, as no external energy is needed. |
|
Types |
Brownian motion, Collective diffusion, Effusion of a gas, Electron diffusion, Facilitated diffusion, Gaseous diffusion, Heat flow, Knudsen diffusion, Momentum diffusion, Osmosis, Photon Diffusion, Reverse Diffusion, Self-diffusion, Surface diffusion |
Reverse Osmosis, Forward Osmosis |
|
Examples |
A molecule of sugar in a glass of water, air freshener in the air, etc. |
Being thirsty after eating something salty, tea bag when soaked absorbs water, absorption of water by plant roots, etc. |
Image Courtesy: okc.cc.ok.us, psifilters.com.au









Comments
I want to ask diffusion occur through which membrane
soumya
Mon, 04/21/2014 - 18:57
Still dont understand it
Tomi
Sat, 01/18/2014 - 19:39
Add new comment