Difference between Molecules and Compounds
Key difference: Atoms are the basic units that all matter is made of. Atoms of different elements come together to make molecules. This happens via a chemical reaction. A molecule is the smallest amount of a chemical substance that can exist. Essentially, a compound is a type of molecule. A molecule can be made up of two or more atoms of the same element or two or more atoms of different elements. However, compounds are molecules that are made up of atoms of different molecule.
 Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. They are so tiny that they cannot even be seen through a microscope. There are many types of atoms. Each atom has a unique name, mass and size. The different types of atoms are called elements. Each atom of any element is made up of protons, neutrons and electrons.
Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. They are so tiny that they cannot even be seen through a microscope. There are many types of atoms. Each atom has a unique name, mass and size. The different types of atoms are called elements. Each atom of any element is made up of protons, neutrons and electrons.
An element is a pure chemical substance that has one or one type of atom, distinguished by its atomic number. The atomic number is derived from the number of protons present in the element’s nucleus. There are a total of 118 elements that have been identified, divided between metal, metalloids and non-metals. Each element has its own set of properties. Most elements are available on the earth, while a few have been developed artificially through nuclear reactions. An element is already in its rawest form and cannot be further broken down. All of the elements can be found in the Periodic Table, listed by atomic number.
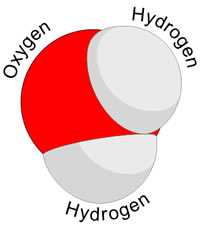
Atoms of different elements come together to make molecules. This happens via a chemical reaction. For example: two hydrogen atoms and one oxygen atom combine to make a water molecule.
A molecule is the smallest amount of a chemical substance that can exist. Such as the smallest amount of water one can have is a molecule of water or H20. It is made of up different atoms together; hence it can be separated back into the different atoms.
A molecule can have very different properties from the elements that it is made of. For example: water behaves very differently than either oxygen or hydrogen, even though it is made up of two hydrogen atoms and one oxygen atom.
Furthermore, an atom cannot exist independently in nature without bonding to something. We will never find just a single oxygen atom or a single carbon atom. It is always bonded with something, such as O2 (oxygen) or CO2 (carbon dioxide). When bonded into a molecule, the molecule can exist independently in nature, which is why we can always find a molecule of oxygen, a molecule of carbon dioxide, a molecule of water (H2O), etc.
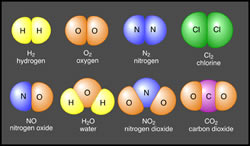 In a molecule, the atoms are stuck together in a particular shape or form. This is mainly depending on the number of bonds that the atom can make. Atoms form molecules by forming chemical bonds with each other. Oxygen atoms always have two bonds with other atoms, carbon atoms always have four bonds with other atoms, and nitrogen atoms always have three bonds with other atoms. Due to this a particular type of molecule always has a particular shape, such as pentagonal, hexagonal, lateral, bi-lateral, etc.
In a molecule, the atoms are stuck together in a particular shape or form. This is mainly depending on the number of bonds that the atom can make. Atoms form molecules by forming chemical bonds with each other. Oxygen atoms always have two bonds with other atoms, carbon atoms always have four bonds with other atoms, and nitrogen atoms always have three bonds with other atoms. Due to this a particular type of molecule always has a particular shape, such as pentagonal, hexagonal, lateral, bi-lateral, etc.
Molecules always tend to group together, their formation depending on their state. Such as in a gaseous state, the molecules tend to be just flying around. In a liquid state, the molecules tend to be loosely grouped together so that they can still move. Whereas in a solid state, the molecules are tightly packed together and can only vibrate.
Molecules are usually represented in a molecular formula. For example: O2, H2O, CO2, C6H12O6 (sugar). The molecular formula is the name of the element followed by the number of atoms of that element in the molecule.
Essentially, a compound is a type of molecule. A molecule can be made up of two or more atoms of the same element or two or more atoms of different elements. However, compounds are molecules that are made up of atoms of different elements. Hence, it can be said that all compounds are molecules, however not all molecules are compounds.
So, hydrogen (H2), oxygen (O2), nitrogen (N2), Water (H2O), carbon dioxide (CO2) and methane (CH4) are all molecules. However, only Water (H2O), carbon dioxide (CO2) and methane (CH4) are compounds, the others are not.
Image Courtesy: wikipedia.org, owensboro.kctcs.edu








Comments
Nice article
ann
Tue, 07/08/2014 - 21:35
Add new comment