Chemistry
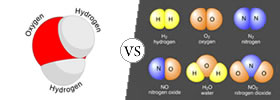
Molecules vs Compounds
|
Atoms are the basic units that all matter is made of. Atoms of different elements come together to make molecules. This happens via a chemical reaction. A molecule is the smallest amount of a chemical... |
Elements vs Atoms
|
An element is a pure chemical substance that has one or one type of atom, distinguished by its atomic number. There are a total of 118 elements that have been identified, divided between metal, metalloids and... |
Atom vs Molecule
|
Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. Each atom of any element is made up of protons, neutrons and electrons. Atoms of different... |
Ionic vs Covalent Bond
|
An ionic bond is a chemical bond between two dissimilar (i.e. a metal and a non-metal) atoms in which one atom gives up an electron to another. A covalent bond is another strong chemical bond. It takes place... |
Atomic mass vs Atomic number
|
Atomic mass is simply the mass of a specific isotope, or the combined mass of the atom’s protons, neutrons and electrons. Atomic number is the number of protons that is found in a nucleus of an element. |